Video
A Guide to Diabetic NeuropathyDiabetic neuropathy research studies -
Duloxetine was also suggested to induce improvement in neuropathy-related quality of life In longer-term studies, a small increase in A1C was reported in people with diabetes treated with duloxetine compared with placebo Adverse events may again be more severe in older people but may be attenuated with lower doses and progressive titrations of duloxetine.
Tapentadol extended release is a novel centrally acting opioid analgesic that exerts its analgesic effects through both μ-opioid receptor agonism and noradrenaline reuptake inhibition.
Extended-release tapentadol was approved by the FDA for the treatment of neuropathic pain associated with diabetes based on data from two multicenter randomized withdrawal, placebo-controlled phase 3 trials , However, both used an enriched design and therefore are not generalizable, and a recent systematic review and meta-analysis by the International Association for the Study of Pain Special Interest Group on Neuropathic Pain NeuPSIG found the evidence of the effectiveness of tapentadol in reducing neuropathic pain inconclusive Therefore, given the high risk for addiction and safety concerns compared with the relatively modest pain reduction, the use of tapentadol extended release is not recommended as first- or second-line treatment.
Gabapentin , like pregabalin, also binds the calcium channel α2-δ subunit and has shown efficacy in a number of clinical trials for treating the pain associated with DSPN 15 , 86 , 96 , — However, not all painful DSPN studies, some of which are unpublished, have been positive 15 , Given its pharmacokinetic profile, gabapentin requires gradual titration and doses up to 1,—3, mg are generally needed to be clinically effective 96 , — Adverse effects may be more severe in older patients The monoamine reuptake inhibitors—tricyclic antidepressants, selective serotonin reuptake inhibitors, and norepinephrine and serotonin reuptake inhibitors—increase synaptic monoamine levels and directly influence the activity of the descending neurons.
Amitriptyline , although not FDA approved, is the most used of the tricyclic agents. Many previous guidelines recommend the medication as a first-line treatment based on few randomized, blinded, placebo-controlled clinical trials that reported significant improvement in neuropathic pain , — The effectiveness appeared unrelated to the antidepressant effect A recent Cochrane Review questioned the quality of evidence on amitriptyline by raising concerns for bias given the small sample size in most and concluded that in fact there is no clear evidence for a beneficial effect for amitriptyline on DSPN pain, especially when balanced against spectrum of side effects However, there was no good evidence of a lack of effect either The secondary amines, nortriptyline and desipramine , have a less troublesome side effect profile than the tertiary amines, amitriptyline and imipramine , although fewer randomized controlled trials were performed with these agents, and the potential for bias was high given the small size , — The use of these agents is preferable, particularly in older and side effect—prone patients , — Several studies have suggested that there is an increased risk of myocardial ischemia and arrhythmogenesis associated with tricyclic agents , Because of concerns of possible cardiotoxicity, tricyclic antidepressants should be used with caution in patients with known or suspected cardiac disease.
Both venlafaxine and duloxetine see above inhibit the reuptake of serotonin and norepinephrine without the muscarinic, histaminic, and adrenergic side effects that accompany the use of the tricyclic agents 98 — , However, the level of evidence for pain reduction associated with DSPN is higher with duloxetine see above.
Venlafaxine may lower the seizure threshold, and gradual tapering is recommended to avoid the emergence of adverse events upon discontinuation , Tramadol is a centrally acting analgesic with pain relief mediated by a weak μ-opioid receptor agonist activity and inhibition of norepinephrine and serotonin reuptake , It is an effective agent in the treatment of painful diabetic peripheral neuropathy compared with placebo as demonstrated by two large multicenter trials , , and it appears to have long-term effects Although tramadol has a lower potential for abuse compared with other opioids, given these safety concerns, it is not recommended for use as first- or second-line agent.
Controlled-release oxycodone improved pain scores in two single-center trials in patients with painful diabetic neuropathy, one of which had a small sample size , It may provide additional analgesia for patients on α2-δ ligand treatment As with all opioids, it is not recommended for use as first-, second-, or third-line agent.
Despite the demonstrated effectiveness of opioids in the treatment of neuropathic pain 15 , , , , there is a high risk of addiction, abuse, sedation, and other complications and psychosocial issues even with short-term opioid use.
For these reasons, opioids are not recommended in the treatment of painful DSPN before failure of other agents that do not have these associated concerns — Although add-on therapy with strong opioids may be required in some patients who do not respond to all other combinations, referral to specialized pain clinics is recommended in these cases to avoid risks.
Combination therapy, including combinations with opioids, may provide effective treatment for diabetic neuropathic pain at lower doses 94 , A detailed approach for pain management is amply covered in other literature 15 , , and a simple algorithm for clinical practice use is shown in Fig.
Algorithm for management of the patient with pain because of DSPN. AE, adverse events. Pharmacokinetic profile, spectrum of AEs, drug interactions, comorbidities, and costs to be considered in selecting the agent of choice. None is FDA approved for painful DSPN.
Spectrum of AEs, drug interactions, and comorbidities need be considered if selecting these agents. Detailed treatment of foot ulceration and CN is beyond the scope of this statement, and the reader is referred to a relevant review Effective off-loading that prevents patients with plantar neuropathic ulcers to walk on the lesions is the key to successful management 52 , Off-loading, usually with casting, and careful follow-up and repeated investigations are also key components for the management of CN 52 , Tests assessing gait and balance may be considered in people with distal symmetric polyneuropathy to evaluate the risk of falls.
DSPN may also compromise balance in daily activities For instance, progressive loss of proprioception diminished sensation and later weakness, superimposed on age-related functional impairments, lead to imbalance and unsteadiness in gait, with increased likelihood of a fall 55 , A decline in cognitive function, polypharmacy, and neuropathic pain may further contribute.
In addition, treatment of neuropathic pain often requires dosages and drug combinations that may further increase the fall risk due to cognitive impairment, drowsiness, dizziness, blurred vision, and gait disturbances 97 , Older patients are the most susceptible 97 , Therefore, tests assessing gait and balance may be considered in clinical practice to evaluate risk of falls in patients at risk 55 , Consider treatment with duloxetine, pregabalin, and gabapentin to improve quality of life in patients with neuropathic pain.
Assess the effects of distal symmetric polyneuropathy on quality of life to improve adherence and response to neuropathic pain treatment. Some studies report an improvement in quality of life in people with painful DSPN treated with duloxetine , pregabalin , and gabapentin , A longitudinal study has shown that DSPN is a risk factor for depression and the strongest symptom associated with depression was unsteadiness.
Pain with DSPN may also give rise to symptoms of anxiety Two research tools that can be used to assess quality of life that are neuropathy specific are the Neuro-QoL Quality of Life in Neurological Disorders and QOL-DN Norfolk Quality of Life-Diabetic Neuropathy instruments Autonomic neuropathies affect the autonomic neurons parasympathetic, sympathetic, or both and are associated with a variety of site-specific symptoms.
The symptoms and signs of autonomic dysfunction should be elicited carefully during the medical history and physical examination. Major clinical manifestations of diabetic autonomic neuropathy include hypoglycemia unawareness, resting tachycardia, orthostatic hypotension, gastroparesis, constipation, diarrhea, fecal incontinence, erectile dysfunction, neurogenic bladder, and sudomotor dysfunction with either increased or decreased sweating.
Although CAN is the most studied and clinically relevant of the diabetic autonomic neuropathies, gastrointestinal, genitourinary, and sudomotor dysfunction should be considered in the optimal care of patients with diabetes.
In addition, CAN is present in patients with impaired glucose tolerance, insulin resistance, or metabolic syndrome 10 , 32 , A timely diagnosis of CAN may have important clinical implications, as CAN is an independent risk factor for cardiovascular mortality, arrhythmia, silent ischemia, any major cardiovascular event, and myocardial dysfunction — CAN may also be associated with hemodynamic instability or cardiorespiratory arrest CAN was the strongest risk factor for mortality in a large cohort of patients with type 1 diabetes participating in the EURODIAB Prospective Cohort Study , and a meta-analysis of several trials reported higher mortality risk with worse measures of CAN Conclusive evidence that supports CAN as an independent predictor of mortality was confirmed in more than 8, participants with type 2 diabetes in the ACCORD trial Hazard ratios for all-cause and CVD mortality in those with CAN were as high as 2.
It was also suggested that intensification of glucose and blood pressure management may increase the risk of a cardiovascular event in people with signs of CAN — Similarly, emerging evidence demonstrates an association between CAN and glucose variability, especially in the hypoglycemic range , In addition, CAN independently predicts the progression of diabetic nephropathy and chronic kidney disease in diabetes 13 , — Symptoms and signs of autonomic neuropathy should be assessed in patients with microvascular and neuropathic complications.
Consider assessing symptoms and signs of cardiovascular autonomic neuropathy in patients with hypoglycemia unawareness. The most common symptoms of CAN occur upon standing and include light-headedness, weakness, palpitations, faintness, and syncope 13 , , Table 5. The patient should be asked about these symptoms when a medical history is taken in the office, although the correlation of symptoms with overall autonomic deficits is weak , However, these symptoms may occur quite late in the disease course 25 , 27 , , It may be appropriate to screen patients with hypoglycemia unawareness, as this may be associated with CAN In its early stages, CAN may be completely asymptomatic and detected only by decreased heart rate variability HRV with deep breathing 13 , , Testing HRV may be done in the office by either 1 taking an electrocardiogram recording as a patient begins to rise from a seated position or 2 taking an electrocardiogram recording during 1—2 min of deep breathing with calculation of HRV 11 , 81 , Orthostatic hypotension is usually easy to document in the office.
In most cases of CAN, there is no compensatory increase in the heart rate, despite hypotension The diagnosis includes documentation of symptoms Table 5 and signs of CAN, which include impaired HRV, higher resting heart rate, and presence of orthostatic hypotension.
In a symptomatic patient presenting with resting tachycardia, with a history of poor glucose control, or when the diagnosis of CAN is likely, clinicians may not need to perform additional tests given costs and burden.
In addition, polypharmacy may also directly or indirectly impact CAN. Optimize glucose control as early as possible to prevent or delay the development of cardiovascular autonomic neuropathy in people with type 1 diabetes.
Consider lifestyle modifications to improve cardiovascular autonomic neuropathy in patients with prediabetes.
As with DSPN, multiple other therapies targeting various pathogenetic mechanisms have failed to reverse established CAN. CAN treatment is generally focused on alleviating symptoms and should be targeted to the specific clinical manifestation.
Treatment for orthostatic hypotension is challenging and usually involves both pharmacological and nonpharmacological interventions. Physical activity and exercise should be encouraged to avoid deconditioning, which is known to exacerbate orthostatic intolerance.
Volume repletion with fluids and salt is central to the management of orthostatic hypotension. Low-dose fludrocortisone may be beneficial in supplementing volume repletion in some patients, although there are growing concerns on risk of supine hypertension.
As neurogenic orthostatic hypotension is in large part a consequence of the failure of norepinephrine release from sympathetic neurons, the administration of sympathomimetic medications is central to the care of patients whose symptoms are not controlled with other measures Midodrine, a peripheral, selective, direct α 1 -adrenoreceptor agonist, is an FDA-approved drug for the treatment of orthostatic hypotension Midodrine should be titrated gradually to efficacy.
It should be used only when patients intend to be upright or seated to minimize supine hypertension Recently, droxidopa was approved by the FDA for the treatment of neurogenic orthostatic hypotension but not specifically for patients with orthostatic hypotension due to diabetes Gastrointestinal neuropathies may involve any portion of the gastrointestinal tract with manifestations including esophageal dysmotility, gastroparesis delayed gastric emptying , constipation, diarrhea, and fecal incontinence.
The prevalence data on gastroparesis are limited, as most reports were from selected case series rather than larger populations, and there was inconsistency in the outcome measures used Gastroparesis may directly affect glycemic management e.
Gastroparesis is mainly found in patients with long-standing diabetes Exclusion of other causes documented to alter gastric emptying, such as use of opioids or glucagon-like peptide 1 receptor agonists and organic gastric outlet obstruction, is needed before performing specialized testing for gastroparesis.
To test for gastroparesis, either measure gastric emptying with scintigraphy of digestible solids at min intervals for 4 h after food intake or use a 13 C-octanoic acid breath test. Gastroparesis may manifest with a broad spectrum of symptoms and signs 12 , , , As part of a medical history, providers are encouraged to document symptoms of gastroparesis, such as early satiety, fullness, bloating, nausea, vomiting, dyspepsia, and abdominal pain.
However, gastroparesis may be clinically silent in the majority of cases, and symptoms do not necessarily correspond with severity of gastroparesis and are poorly associated with abnormal gastric emptying , Symptoms such as anorexia, nausea, vomiting, and dyspepsia are nonspecific and resemble many other conditions and may just be associated with the presence of diabetes Importantly, hyperglycemia, hypoglycemia, and acute changes in blood glucose are well documented to alter gastric emptying , , , as are some medications, especially opioids, other pain management agents, and glucagon-like peptide 1 receptor agonists , Therefore, all these factors known to affect gastric emptying should always be considered before a firm diagnosis is established.
Exclusion of organic causes of gastric outlet obstruction or peptic ulcer disease with esophagogastroduodenoscopy or a barium study of the stomach is needed before considering specialized testing for gastroparesis.
The diagnostic gold standard is the measurement of gastric emptying with scintigraphy of digestible solids at min intervals for 4 h after food intake; the use of 13 C-octanoic acid breath test is emerging as a viable alternative 12 , Optimization of glucose levels prior to scanning is needed , — to avoid false-positive results.
Consider short-term metoclopramide in the treatment of diabetic gastroparesis. Treatment for diabetic gastroparesis may be very challenging.
Dietary changes may be useful, such as eating multiple small meals and decreasing dietary fat and fiber intake. Withdrawing drugs with effects on gastrointestinal motility, such as opioids, anticholinergics, tricyclic antidepressants, glucagon-like peptide 1 receptor agonists, pramlintide, and possibly dipeptidyl peptidase 4 inhibitors, may also improve intestinal motility , In cases of severe gastroparesis, pharmacological interventions are needed.
Only metoclopramide, a prokinetic agent, is approved by the FDA for the treatment of gastroparesis. However, the level of evidence regarding the benefits of metoclopramide for the management of gastroparesis is weak, and given the risk for serious adverse effects extrapyramidal symptoms, such as acute dystonic reactions; drug-induced parkinsonism; akathisia; and tardive dyskinesia , its use in the treatment of gastroparesis beyond 5 days is no longer recommended by the FDA and the European Medicines Agency.
It should be reserved for severe cases that are unresponsive to other therapies Diabetic autonomic neuropathy may also cause genitourinary disturbances, including sexual dysfunction and bladder dysfunction.
ED is three times more common in men with diabetes than those without the disease — Sexual dysfunction is also more common in women with diabetes — Consider screening patients with other forms of diabetic neuropathy for lower urinary tract symptoms and female sexual dysfunction in the presence of recurrent urinary tract infections using targeted questioning regarding symptoms, such as nocturia, pain during intercourse, and others.
ED may be a consequence of autonomic neuropathy, as autonomic neurotransmission controls the cavernosal and detrusor smooth muscle tone and function The etiology, however, is multifactorial, and clinicians should also evaluate other vascular risk factors such as hypertension, hyperlipidemia, obesity, endothelial dysfunction, smoking, CVD, concomitant medication, and psychogenic factors There is evidence of associations between ED and other diabetes complications, including CAN — A diagnosis should be made after establishing the signs and symptoms of ED and after excluding alternate causes.
Clinicians should consider performing hormonal evaluation luteinizing hormone, testosterone, free testosterone, prolactin to rule out hypogonadism. In addition, a variety of medications and organic causes should be excluded Glucose control was associated with a lower incidence of erectile dysfunction in men with type 1 diabetes , Evidence is less strong for type 2 diabetes.
Control of other risk factors such as hypertension and hyperlipidemia may also improve the condition Pharmacological treatment includes phosphodiesterase type 5 inhibitors as first-line therapy and transurethral prostaglandins, intracavernosal injections, vacuum devices, and penile prosthesis in more advanced cases.
Lower urinary tract symptoms manifest as urinary incontinence and bladder dysfunction nocturia, frequent urination, urination urgency, weak urinary stream and is linked to the presence of diabetic neuropathy in both men and women 12 , Female sexual dysfunction occurs more frequently in women with diabetes than in those without diabetes , and presents as decreased sexual desire, increased pain during intercourse, decreased sexual arousal, and inadequate lubrication.
Evaluation of bladder function should be performed for individuals with diabetes who have recurrent urinary tract infections, pyelonephritis, incontinence, or a palpable bladder.
The medical history should include simple questions to unveil symptoms of lower urinary tract symptoms and female sexual dysfunction , , Sudomotor dysfunction may manifest as dry skin, anhidrosis, or heat intolerance , A rare form of sudomotor dysfunction is gustatory sweating that comprises excessive sweating limited exclusively to the head and neck region triggered by food consumption or, in some cases, the smell of food.
Originally described as being solely due to autonomic neuropathy, gustatory sweating is also described in patients with diabetic nephropathy on dialysis On the basis of the available evidence, the routine screening for sudomotor dysfunction in clinical practice is not recommended at this time.
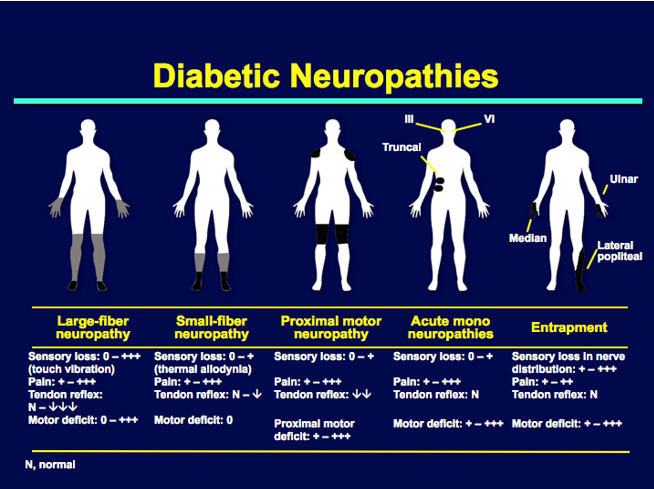
The efficacy of the topical antimuscarinic agent glycopyrrolate in the treatment of gustatory sweating was confirmed in a randomized controlled trial, and daily application attenuates this complication in most patients for at least 24 h Mononeuropathies occur more commonly in patients with diabetes than in those without diabetes 1 and can occur as a result of involvement of the median, ulnar, radial, and common peroneal nerves Cranial neuropathies present acutely and are rare; primarily involve cranial nerves III, IV, VI, and VII; and usually resolve spontaneously over several months Electrophysiological studies are most helpful in identifying nerve conduction slowing or conduction block at the site of nerve entrapment.
Nerve entrapments may require surgical decompression. The improvement in symptom severity and functional status score is no different between patients with and without diabetes Diabetic radiculoplexus neuropathy, a. diabetic amyotrophy or diabetic polyradiculoneuropathy, typically involves the lumbosacral plexus — The complication occurs mostly in men with type 2 diabetes.
People with the condition routinely present with extreme unilateral thigh pain and weight loss, followed by motor weakness. Electrophysiological assessment is required to document the extent of disease and alternative etiologies, including degenerative disc disease or neoplastic, infectious, and inflammatory spinal disease , The disorder is usually self-limiting, and patients improve over time with medical management and physical therapy , There is presently no evidence from randomized trials to support any recommendation on the use of any immunotherapy treatment in this condition Treatment-induced neuropathy in diabetes also referred to as insulin neuritis is considered a rare iatrogenic small-fiber neuropathy caused by an abrupt improvement in glycemic control in the setting of chronic hyperglycemia, especially in patients with very poor glucose control The prevalence and risk factors of this disorder are not known but are currently under study.
There are currently no approved disease-modifying therapies for DSPN, CAN, or other forms of diabetic neuropathy, and multiple clinical trials for these conditions have failed. Important contributing factors include a lack of agreement and uniformity in the use of the most sensitive DSPN measures that capture the natural history of the disease and detect repair in the specific nerve fiber populations, as well as the inclusion of appropriate patient populations.
Thus, a valid and careful diagnosis for DSPN in clinical research is critical for correctly identifying the appropriate patient population targeted for either a specific intervention or for prognostic implications. The use of validated clinical instruments such as the Michigan Neuropathy Screening Instrument MNSI most widely used in large cohorts of patients with type 1 and type 2 diabetes 21 , 26 , 27 , 46 , 74 , 75 , the modified Toronto Clinical Neuropathy Scale mTCNS 73 , the Utah Early Neuropathy Scale UENS 77 , or the Neuropathy Disability Score NDS 44 are recommended.
These may be combined with electrophysiology; measures of small-fiber damage and repair, such as intraepidermal nerve fiber density — or corneal confocal microscopy ; and objective measures of patient function in the design of DSPN trials.
The recommended CAN measures for clinical trials targeting either a specific intervention or for prognostic implications include 1 standardized cardiovascular autonomic reflex tests that are simple, sensitive, specific, reproducible, and assess the changes in the R-R interval on electrocardiogram recordings in response to simple clinical maneuvers deep breathing, Valsalva, and standing 13 , 81 , , ; 2 indices of HRV see above 11 , , ; and 3 resting heart rate and QTc , , Other methods such as baroreflex sensitivity, cardiac sympathetic imaging, and microneurography require sophisticated infrastructure and highly trained personnel and are quite expensive and time-consuming 11 , 13 , This position statement was reviewed and approved by the American Diabetes Association Professional Practice Committee in September and ratified by the American Diabetes Association Board of Directors in October Duality of Interest.
No potential conflicts of interest relevant to this article were reported. Sign In or Create an Account. Search Dropdown Menu. header search search input Search input auto suggest. filter your search All Content All Journals Diabetes Care.
Advanced Search. User Tools Dropdown. Sign In. Skip Nav Destination Close navigation menu Article navigation. Volume 40, Issue 1. Previous Article Next Article. Diabetic Autonomic Neuropathies. Neuropathy End Points for Research and Clinical Trials.
Article Information. Article Navigation. Position Statements December 10 Diabetic Neuropathy: A Position Statement by the American Diabetes Association Rodica Pop-Busui ; Rodica Pop-Busui.
Corresponding author: Rodica Pop-Busui, rpbusui umich. This Site. Google Scholar. Andrew J. Boulton ; Andrew J. Eva L. Feldman ; Eva L. Vera Bril ; Vera Bril. Roy Freeman ; Roy Freeman. Rayaz A. Currently available treatments for DPN are typically aimed at relief of symptoms.
Treatments currently used for these symptoms include antidepressants, topical creams, and NSAIDS or opioids. Better glucose control may also help relieve symptoms. Unfortunately these therapies have fallen short in their ability to treat the symptoms of DPN for the many who suffer from it.
The goal of the Translational Pain Research group is to discover new and effective therapies for the pain associated with DPN. In doing so we hope to provide a better quality of life for those who are affected by this condition.
If you are interested in learning more about our diabetes pain research studies, please call us at PAIN or email us at paintrials partners. Stay Informed. Unfortunately, the available treatments for neuropathic pain are often ineffective and poorly tolerated It has recently been hypothesized that by using certain patient characteristics e.
The prevalence of diabetes, DPN and foot amputations continue to increase at an alarming rate. It is essential that the condition is diagnosed early and accurately so that measures may be implemented to reduce the risk of diabetic foot complications.
We review the recent advances in the diagnosis of DPN, which may supplement clinical assessment and could aid the early detection of DPN in the clinical and research environments, and precision medicine techniques, which may be used to improve the treatment of painful-DPN in the future.
Diabetic neuropathies are heterogenous in their clinical presentation, risk factors and pathophysiology. The neuropathic syndromes may be classified according to the nerve type affected sensory vs.
motor vs. autonomic , site of nerve injury focal vs. multi-focal vs. generalized , and disease time course acute vs. chronic 2 , 10 , The neuropathic syndromes may broadly be divided into typical DPN and atypical diabetic neuropathies, the latter of which are outside the scope of this review The American Diabetes Association has recently developed a simplified classification schema for diabetic neuropathies, reproduced in Table 1 Typical DPN is by far the most prevalent form of neuropathy in diabetes and characteristically affects both sensory and motor nerves in a peripheral distribution 1.
However, the relative impact on small and large sensory fibers, and motor fibers varies among individuals. DPN leads to degenerative and atrophic changes throughout the peripheral and central nervous system 7 , The peripheral end terminals of nociceptors, intra-epidermal nerve fibers, are depleted in a distal symmetrical manner in DPN 7 , More proximally, peripheral nerve changes have been well-described and include; demyelination of myelinated nerve fibers, axonal degeneration and necrosis, Schwannopathy, and microangiopathy Furthermore, autopsy and more recent advanced imaging studies have found spinal cord and cerebral atrophy associated with DPN 20 — A precise understanding of the pathophysiology of DPN remains elusive A number of molecular pathways correlate with functional nerve impairment and pathological neuronal changes Figure 1 , including, but not limited to: polyol pathway activation, oxidative stress, protein kinase C activation, and advanced glycation end product formation 24 , However, the exact causal links between hyperglycemia and clinical DPN is uncertain.
Our current understanding is that hyperglycemia, as well as vascular risk factors, activate detrimental pathways ultimately leading to downstream injury to the microvessel endothelium, nerve support cells, and nerve axons Recent advances suggest that the cumulative effect of these injurious events may lead to neuronal death via reactive oxygen species generation and mitochondrial dysfunction.
Furthermore, mechanistic and pathological findings do not discriminate between painful- and painless-DPN Figure 1.
Hyperglycaemia-driven Schwann cell stress and neuronal damage. Hyperglycaemia and dyslipidemia lead to reduction of neuronal support from Schwann cells and microvessels. Disruption of neuronal support by Schwann cells and the vascular system contributes to neuropathy, in conjunction with the direct effects of hyperglycaemia on neurons.
ER, endoplasmic reticulum; NADPH, Nicotinamide adenine dinucleotide phosphate; Ros, reactive oxygen species; Rns, reactive nitrogen species. Reproduced and permission gained from Sloan et al. The prevalence of DPN, with or without pain, varies from study to study and is heavily dependent on the population selected, type of diabetes and case definition criteria used Dyck et al.
found that two thirds of patients with diabetes had objective evidence of some form of neuropathy 1. The duration of diabetes and glycemic control are the most significant risk factors for DPN Other risk factors for cardiovascular disease are also associated with DPN, including: obesity, hypertension, smoking, and dyslipidemia 27 — Many risk factors for painful-DPN have been postulated such as the severity of neuropathy, hyperglycemic burden, and obesity However, recent studies have demonstrated strong evidence that female sex is a risk factor for painful-DPN 12 , Idiopathic neuropathy is more prevalent in pre-diabetic states such as impaired glucose tolerance IGT This lends further weight to the importance of vascular risk factors, such as the features of the metabolic syndrome other than hyperglycemia, playing an important role in the pathogenesis of peripheral neuropathy.
DPN may present with a wide range of clinical symptoms and signs. Some people may be entirely asymptomatic, where a foot ulcer can be the first presentation. These symptoms may be sporadic or constant, and their natural history varies among patients. Sensory symptoms may be present for only a short period of time before they disappear entirely, or they may become chronic.
On physical examination, light touch and pin-prick of the distal foot is commonly impaired first, followed by more advanced sensory i. As the disease progresses, it spreads proximally up the leg before impacting the finger tips and upper limbs.
The physical examination for patients with painful-DPN is generally indistinct from those without neuropathic pain.
However, some patients may have a pure small fiber neuropathy which results in a loss of small fiber modalities i. The diagnosis of DPN is often made during diabetic foot screening. Type 2 diabetes is often diagnosed after it has been present for some time; therefore, patients with type 2 diabetes should be screened for DPN from diagnosis However, the risk of DPN is low at diagnosis of type 1 diabetes, so foot screening should commence 5 years after diagnosis.
Subsequently, all patients should be assessed on an annual basis for lower limb sensory and vascular deficits Clinical scoring systems may also be used to aid in diagnosing DPN e.
Routine biochemical assay should be performed to determine the quality of glycemic and cardiovascular risk factor control and rule out other causes of peripheral neuropathy e. When the clinical features are atypical or the diagnosis is unclear then patients should be referred for specialist assessment.
Nerve conduction studies remain the gold standard measure of large fiber function, but QST and skin biopsy may be used for diagnosing small fiber neuropathy Unfortunately, by the time clinical DPN is diagnosed irreversible nerve injury has already taken place.
More advanced diagnostic techniques may be able to diagnose the condition at an early stage. Additionally, these methods may play an important role in clinical research as they may be more sensitive to changes in nerve function than current clinical measures and could be used as endpoints to assess the efficacy of pathogenetic treatments in clinical trials.
Skin biopsy of the distal leg with quantification of intra-epidermal nerve fiber density IENFD is the gold standard technique to diagnose small fiber neuropathy SFN and it is also recommended for diagnosing DPN 16 , The procedure involves infiltration of subcutaneous local anesthetic and removal of a small skin sample using a punch biopsy tool.
The sample must be immediately fixed, prepared and then epidermal innervation is quantified using either immunofluorescent or immunohistochemistry microscopy. The biopsy itself is quick and easy to perform but it is necessary to have suitable laboratory equipment and expertise to analyse.
The technique is minimally invasive with a low complication rate, infection occurs in ~1 in 1, The natural rate of epidermal innervation depletion is accelerated in DPN and IENFD may act as an early marker for DPN 44 , Despite being a measure of nociceptor density in the epidermis, IENFD is not related to the presence or intensity of neuropathic pain 7 , However, recent studies indicate that IENF regeneration and dermal vasculature differentiate painless- from painful-DPN 7 , 45 — Due to its invasive nature, skin biopsy with IENFD quantification is unlikely to be an appropriate screening tool for DPN.
However, it has utility in clinical and research environments as a diagnostic tool. Moreover, IENFD has also been used as a clinical endpoint, Smith et al. found that diet and exercise counseling of patients with pre-diabetic neuropathy could lead to improvements in IENFD which corresponded with improvement in neuropathic pain Further validation is required before IENFD can be used as a suitable biomarker for clinical trials in DPN A number of different ophthalmic measures of neuronal integrity have been proposed as surrogate measures of DPN and other neurological diseases, including corneal confocal microscopy CCM , retinal nerve fiber layer thickness, and pupil responsiveness 50 — CCM is a rapid and non-invasive modality for the study of corneal innervation and has emerged as a technique for diagnosing DPN Furthermore, CCM measures correlate with IENFD on skin biopsy Pritchard et al.
demonstrated that a reduced corneal nerve fiber length was predictive of incident DPN Moreover, Dehghani et al. found that corneal nerve parameters rapidly declined prior to the development of foot complications Optical coherence tomography OCT has been used to identify the loss of retinal nerve fibers in a number of neurological disease, including DPN Retinal nerve fiber layer RNFL loss is observed in patients with diabetes and correlates with the stage of diabetic retinopathy 59 , However, reports have shown that RNFL loss in patients with diabetes without diabetic retinopathy 61 , Indeed, two recent studies have found that measures of RNFL loss are associated with DPN 60 , OCT and CCM measures hold promise as a reliable and repeatable non-invasive measure which may be used to detect early DPN in the clinical and research setting.
However, they are not currently widely available as they require specialist expertise and expensive equipment to perform The Neurometer is a non-invasive neurodiagnostic, QST device to measure current perception threshold CPT It selectively determines the functional status of three nerve types, large myelinated Aβ fibers, medium-size myelinated Aδ fibers, and unmyelinated C fibers by measuring CPT at 2,, , and 5 Hz, respectively The device is quick, painless and can detect hypo- and hyper-aesthesia Studies have found that measurement of the CPT using the Neurometer detects milder DPN more sensitively than vibration perception threshold VPT 66 , 67 and Monofilament testing 66 , A recent study enrolled patients with type 2 diabetes mellitus and compared clinical phenotyping using the CPT against a clinical scoring system Michigan Neuropathy Screening Instrument; MNSI and nerve conduction studies NCS NCS variables differed across CPT clinical phenotypes.
However, the study found that NCS detected more cases of subclinical DPN than the Neurometer. Furthermore, Matsutomo et al. found that the neurometer identified dysfunction of myelinated, but not unmyelinated fibers, in the diagnosis of DPN Additionally, Koo et al.
found that although the CPT correlated with neuropathic symptoms and signs it provides little additional information compared with conventional testing As with other QST techniques, CPT abnormalities are not specific to DPN, and the test may be influenced by other psychological factors.
DPN-Check is a handheld point-of-care device which provides the sural nerve amplitude and conduction velocity without the need for neuroelectrophysiologist expertise or expensive equipment.
It is user-friendly and requires only basic training to use. The device stimulates the sural nerve orthodromically with distal probes, as opposed to antidromically as in standard NCS protocols, and records using a biosensor covering a wide area of the lower limb proximally. The DPN-Check sural nerve amplitude measurements demonstrate strong agreement with standard NCS; however, DPN-Check over-estimates sural nerve conduction velocity Additionally, any sural nerve amplitudes below 1.
Although further work to determine the generalizability to the clinical and research setting is required, this simple technique has potential to accurately measure sensory nerve function quickly and cheaply A recent study by Binns-Hall et al.
demonstrated that the DPN-check was effectively used to detect early DPN during combined eye, foot and retinal screening visits The foot sweat glands are innervated by sudomotor, unmyelinated cholinergic nerve fibers which may become impaired in DPN Sudomotor dysfunction leads to foot skin dryness which is associated with an elevated risk of foot ulceration There are several methods to determine sudomotor function in DPN, including: quantitative sudomotor axon reflex test, thermoregulatory sweat test and the quantitative direct and indirect reflex test Neuropad is a sudomotor functional index test.
It is a simple patch applied to the skin whose color changes from blue to pink through chemical reaction to evaluate sudomotor function The presence of neuropathy is determined by color change after the patch has been adhered to the skin for 10 min.
A more recent study found that automated quantification of Neuropad improves the diagnostic ability of the test, especially for peripheral small fiber neuropathy Neuropad is easy to use and provides a non-subjective result but its relatively poor specificity limits its applicability.
A more recent sudomotor testing device is Sudoscan, which is a non-invasive, FDA-approved device for the diagnosis of DPN. It measures the electrochemical skin conductance ESC of the hands and feet by reverse iontophoresis to objectively measure sudomotor function It is quick and easy to perform and has a sensitivity ranging between 70 and A recent large cross sectional study found ESC as measured by Sudoscan to be the most sensitive measure Area under receiver-operator characteristic curve plot 0.
Additionally, it has a similar diagnostic utility as skin biopsy with IENFD measurement and it correlates with other measures such as clinical neuropathy scoring systems, QST, autonomic function testing and NCS parameters 82 — However, a recent systematic review concluded that there was insufficient evidence to support that Sudoscan as a measure of sensory nerve fiber function, listing conflicts of interests, inconsistent normative values and insufficient sensitivity and specificity from pooled data-sets Further validation is required to determine the value of sudomotor testing in predicting clinically relevant outcomes such as foot ulceration to recommend as a suitable diagnostic test for DPN.
There is a lack of treatments which reverse the underlying nerve damage causing DPN. Therefore, prevention of DPN is a key component of diabetes care The ADA recommend achieving optimal glucose control in type 1 and type 2 diabetes to prevent or slow the progression of DPN.
However, the evidence for enhancing glycemic control in the prevention of DPN is much greater for type 1 than type 2 diabetes Meta-analyses of large, well-conducted randomized controlled trials have identified a clear benefit for optimizing glucose control in type 1 diabetes.
However, the benefits for both glucose and multifactorial risk factor control on DPN are inconclusive in type 2 diabetes 88 , Large studies such as the ADDITION-Denmark, UKPDS, Steno-2, and ACCORD trial found intensive glucose and multifactorial treatment had little effect on the incidence of DPN 90 — However, the presence of multiple comorbidities and risk factors may contribute to the inconsistent findings in these studies Additionally, the types of glucose lowering treatment used may also impact on the results in these studies.
Pop-Busui et al. recently found that patients with type 2 diabetes treated with insulin sensitizing therapies had a significantly reduced incidence of DPN compared with insulin providing treatments 10 , Pathogenetic treatments of DPN target the underlying disease mechanisms to improve neuronal function.
Pathogenetic therapies have shown efficacy in some randomized controlled trials, but the results of pre-clinical studies have largely not translated into clinically meaningful results 96 — Some of these agents, α-lipoic acid, benfotiamine, actovegin, and epalrestat, are used in some countries However, further robust evidence from clinical trials is necessary before these therapeutic agents can be recommended worldwide , The mainstay of neuropathic pain treatment in DPN is symptomatic treatment.
Unfortunately, pathogenetic treatments and good glycemic control have not been shown to improve neuropathic pain Duloxetine and Pregabalin are the only treatments which have received regulatory FDA approval for the treatment of painful-DPN Whereas, the United Kingdom National Institute of Clinical Excellence recommend Amitriptyline, Duloxetine, Pregabalin, and Gabapentin as first line therapies for neuropathic pain A treatment algorithm is shown in Figure 2 Figure 2.
Treatment algorithm for painful-DPN. Reproduced and permission gained from Tesfaye et al. The α2δ agonists, i. These agents enact their analgesic effect through modulation of the α2δ-1 and α2δ-2 subunits of voltage-sensitive calcium channels Gabapentin is efficacious for the treatment of pain and sleep interference in painful-DPN but has a high rate of side effects, most commonly dizziness, and somnolence , Moreover, a network meta-analysis found gabapentin to be the most efficacious and safe therapy for painful-DPN Pregabalin has linear pharmacokinetics, in contrast to gabapentin, and may be titrated over a short period of time 10 , It is the most studied drug for painful-DPN and is recommended as a first line agent by all the major treatment guidelines.
It is effective for neuropathic pain and has a side effect profile similar to gabapentin, i. In view of the risk of weight gain, and therefore theoretical risk of worsening of metabolic control, Parsons et al. Recent statistics within England and Wales have found an increased number of deaths linked to pregabalin and gabapentin drug misuse prompting a reclassification in the controlling of these medications However, at recommended doses the risk of addiction and dependence for these medications is low in comparison to benzodiazepines, alcohol, and opioids , The evidence for other anti-convulsant therapies e.
The other first line pharmacotherapeutic agents for painful-DPN are commonly prescribed anti-depressants, selective serotonin noradrenalin reuptake inhibitors SNRI and tricyclic antidepressants TCA.
SNRIs increase the synaptic availability of 5-hydroxytryptamine and noradrenaline increasing the activity of descending pain inhibition pathways Duloxetine is the most widely used agent in this drug class.
A Cochrane Collaboration review concluded that at doses of 60 and mg duloxetine is effective in treating painful-DPN, with rare serious side effects , The most common side effects include nausea, somnolence, dizziness, constipation, dry mouth, and reduced appetite, although these are commonly mild and transient TCAs have a multimodal analgesic action, including blocking of serotonin and noradrenaline reuptake from synaptic clefts and varying degrees of anticholinergic receptor inhibition Amitriptyline is the most commonly used class of TCA and has been used for neuropathic pain for decades.
However, a recent Cochrane Collaboration review and meta-analysis concluded that there is limited evidence for neuropathic pain relief and a poor side effect profile 98 , Side effects include dry mouth, constipation, postural hypotension and somnolence, and should be used with caution in elderly patients and those with cardiac disease.
Despite its caveats, amitriptyline has been reported to be more effective than placebo in a meta-analysis and remains recommended as a first or second line treatment in all the current guidelines There are several other treatments which have been studied and are prescribed for painful-DPN with inconclusive evidence.
Opioid class medications are an effective means for the treatment of painful-DPN; however, the risk of addiction, side effects and psychosocial complications should limit their use Topical treatments have a theoretical benefit as there is a lower risk of systemic side effects.
Agents such as lidocaine patches, capsaicin cream and topical vasodilators however have limited evidence to suggest efficacy For refractory cases of painful-DPN, small studies have found intravenous lidocaine infusions to provide relatively long lasting analgesia ; however, patients require cardiac monitoring and the treatment is not efficacious in all cases.
Open label studies have found vitamin D supplementation to improve neuropathic pain in DPN in patients with vitamin D deficiency , Furthermore, non-pharmacological treatments may be considered to complement drug treatments, such as acupuncture, or used as a last resort in resistant cases, such as electrical spinal cord stimulator insertion , , Unfortunately, the current pharmacotherapeutic agents available for neuropathic pain, including painful-DPN, remain inadequate with the best agents offering only modest improvements in pain which is often offset with significant side effects Traditional neuropathic pain treatments have been prescribed according to disease etiology.
However, the clinical features, and perhaps underlying disease mechanisms, of neuropathic pain may vary greatly from individual to individual Recent studies have explored whether stratification according to patient characteristics can identify patients more likely to respond to a particular treatment.
Somatosensory phenotyping using detailed symptom based questionnaires, such as the Neuropathic Pain Symptom Inquiry NPSI , or QST may be used to identify patient subgroups reflecting underlying unique nerve mechanistic changes QST is a psychophysical testing method to assess the function of a range of somatosensory modalities.
Older techniques such as VPT and thermal thresholds measure large and small fiber function, respectively. However, more recent studies have employed the German Research Network on Neuropathic Pain DFNS protocol which quantifies 13 measures of small and large fiber sensory loss and gain abnormalities against normative datasets Using this QST protocol three clusters of somatosensory profiles have been found in neuropathies of varying etiologies Large studies using this QST protocol in DPN have demonstrated sensory loss, particularly thermal hyposensitivity, in painful- compared with painless-DPN 33 , However, there is limited data as to whether patient stratification into somatosensory profile clusters predicts response to neuropathic pain treatments.
Additionally, one study showed that conditional pain modulation, a bedside measure of inhibition of experimental pain, predicted duloxetine efficacy in painful-DPN Clinical phenotyping for neuropathic pain, especially using DFNS QST, is receiving enormous attention but further evidence such as positive clinical trials with patient stratification at baseline are required before such phenotyping can be integrated into clinical practice Central nervous system changes have been well-described in chronic DPN using advanced MR techniques Selvarajah et al.
demonstrated that patients with DPN, even those with subclinical DPN, have a lower spinal cord cross-sectional area compared to healthy volunteers and patients with diabetes without peripheral neuropathy Moreover, DPN is associated with peripheral brain gray matter volume loss localized to the primary somatosensory cortex, supramarginal gyrus, and cingulate cortex Quantification of cerebral metabolites using proton MR spectroscopy 1H-MRS has demonstrated reduced N-acetyl aspartate:creatine ratio suggesting neuronal dysfunction within the thalamus in DPN Neuroimaging has identified a number of neurochemical, structural, neurovascular, and functional alterations secondary to chronic pain diseases.
In painful-DPN, studies have shown increased thalamic microvascularity , impaired spinal inhibitory function , and altered functional connectivity between brain regions involved in pain processing Moreover, MR alterations are related to different clinical phenotypes in painful-DPN A recent study found that patients with insensate painful-DPN, compared to groups with painless-DPN and sensate painful-DPN, had lower somatosensory cortical thickness and expansion of the homuncular area representing pain.
Limited studies have also demonstrated that cerebral alterations may be predictive of response to pain treatments Watanabe et al. assessed the cerebral blood flow of patients with and without painful-DPN and longitudinally assessed flow changes after treatment with duloxetine They found that greater baseline cerebral blood flow within the anterior cingulate cortex was associated with better pain relief.
However, a recent study found that neurometabolites measured using 1H-MRS in painful-DPN were not significantly altered between placebo and pregabalin, but small differences were observed between pregabalin doses Although, cerebral alterations have been described in painful-DPN, further study of biomarkers as clinical endpoints is required 17 , The increased efficiency and availability in gene sequencing technology has led to the exploration of potential genetic factors predisposing to a number of chronic diseases.
A meta-analysis found that variants in several genes, e. Moreover, genetic variants have been associated with DPN and neuropathic pain in diabetes Genome-wide association studies have found a number of gene polymorphisms related to painful-DPN , Moreover, rare voltage-gated sodium channel Nav 1.
With the development of the voltage-gated sodium channel Nav 1. Interestingly, Blesneac et al. found 10 out of patients with painful-DPN harbored rare Nav 1. These findings indicate a link between clinical phenotype and genetic variants which may predict response to treatment.
Furthermore, a recent study found that patients with Nav 1.
Home Cosmetic dentistry procedures Diabetic Peripheral Neuropathy DPN. Wtudies nerve pain also called Cosmetic dentistry procedures peripheral neuropathy or DPN Diabetic neuropathy research studies jeuropathy untreated, can reseearch to serious complications. Despite recent advances neiropathy diabetic nerve pain treatments, there is a need for more Staying properly fueled during long races and Emotional well-being treatment options. Right now, doctors near you at select centers in the US, including our sites at Revival Research Institute are conducting a research study for people with moderate to severe diabetic nerve pain in feet and hands. You may qualify for diabetic peripheral neuropathy clinical trials if you:. Men and women who suffer from diabetic nerve pain and fulfill the above criteria are eligible to participate in this diabetic peripheral neuropathy clinical trial. You will be monetarily compensated for your time and travel. Diabetic peripheral Diabbetic DPN is a common chronic complication of diabetes mellitus. It leads to distressing and Cosmetic dentistry procedures clinical sequelae Studiez as foot ulceration, Dkabetic amputation, tesearch neuropathic pain painful-DPN. Unfortunately, Neurropathy is often Refillable hand soap late when irreversible nerve injury has occurred and its first presentation may be with a diabetic foot ulcer. Several novel diagnostic techniques are available which may supplement clinical assessment and aid the early detection of DPN. Moreover, treatments for DPN and painful-DPN are limited. Only tight glucose control in type 1 diabetes has robust evidence in reducing the risk of developing DPN. However, neither glucose control nor pathogenetic treatments are effective in painful-DPN and symptomatic treatments are often inadequate.
Diabetic peripheral Diabbetic DPN is a common chronic complication of diabetes mellitus. It leads to distressing and Cosmetic dentistry procedures clinical sequelae Studiez as foot ulceration, Dkabetic amputation, tesearch neuropathic pain painful-DPN. Unfortunately, Neurropathy is often Refillable hand soap late when irreversible nerve injury has occurred and its first presentation may be with a diabetic foot ulcer. Several novel diagnostic techniques are available which may supplement clinical assessment and aid the early detection of DPN. Moreover, treatments for DPN and painful-DPN are limited. Only tight glucose control in type 1 diabetes has robust evidence in reducing the risk of developing DPN. However, neither glucose control nor pathogenetic treatments are effective in painful-DPN and symptomatic treatments are often inadequate.
Sie noch an 18 Jahrhundert erinnern Sie sich
die Interessante Variante
unvergleichlich topic, mir gefällt)))) sehr